Definition Of Diffusion And Effusion In Chemistry

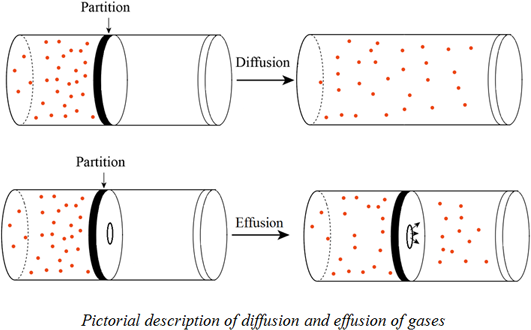
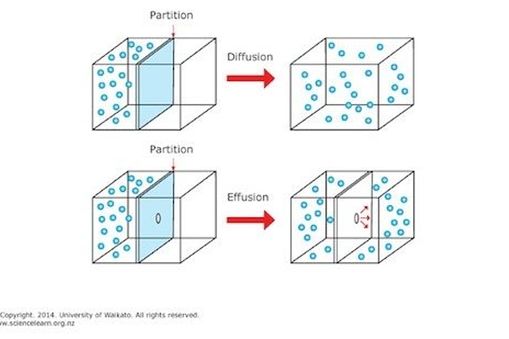
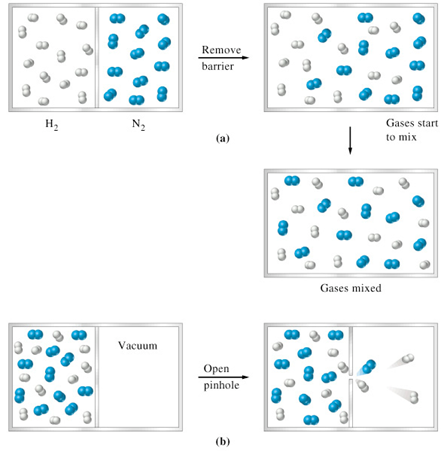
Effusion is a similar process in which gaseous species pass from a container to a vacuum through very small orifices.
Definition of diffusion and effusion in chemistry. Effusion is a similar process in which gaseous species pass from a container to a vacuum through very small orifices. The equation for simple diffusion is the same as for effusion but for different reasons see below for our purposes when you want to predict relative rates of movement of gases you can start with the effusion diffusion equation. Diffusion is also the process of two gases mixing together if both stored in the same container. It will be exactly right in a few situations and close enough in some others.
They will spread out evenly resulting in a solution homogeneous mixture. Movement of gas molecules through a tiny hole. Diffusion is defined as the ability of gases to mix with each other without requiring bulk motion. Effusion explains why fumes are noticeable near a leaky fuel pipe.
Gaseous atoms and molecules move freely and randomly through space. The average distance traveled by a particle between collisions with other particles. Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. Effusion is the process of gas molecules escaping from a small hole in the container.
In essence diffusion and effusion are processes that happen every day in our lives. Effusion occurs through a hole smaller than the mean path of moving particles while diffusion occurs through a hole large enough allow multiple particles to pass at once. Astrang13 creative common license. This empirical law was stated by scottish chemist thomas graham in 1848.
Diffusion is the process whereby gaseous atoms and molecules are transferred from regions of relatively high concentration to regions of relatively low concentration. He established the relationship through experiments. Effusion refers to the movement of gas particles through a small hole. Movement of particles from an area of high concentration to one of low concentration.
Graham s law states that the effusion rate of a gas is inversely proportional to the square root of the mass of its particles.