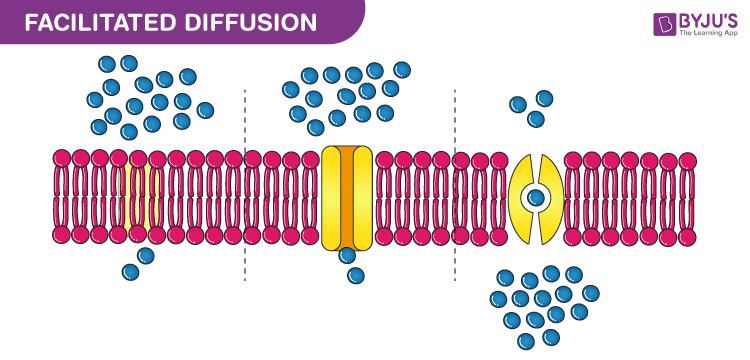
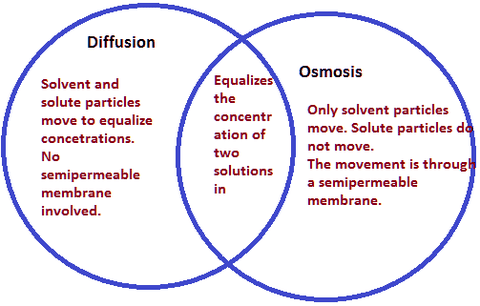
The Best Definition For Diffusion

It is how substances.
The best definition for diffusion. An intermingling of molecules ions etc resulting from random thermal agitation as in the. Diffusion is the net movement of anything for example atom ions molecules from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in concentration. Diffusion process resulting from random motion of molecules by which there is a net flow of matter from a region of high concentration to a region of low concentration.
The act or process of diffusing or being diffused. Diffusion is an important process for living things. The process of rapid random movement of the particles of a liquid or gas which eventually form a uniform mixture. The random thermal motion of atoms molecules clusters of atoms etc in gases liquids and some.
Diffusion is the net passive movement of molecules or particles from regions of higher to regions of lower concentration. Movement of molecules from an area of their higher concentration to an area of their lower concentration diffusion is the net movement of molecules or ions from a region of their high. How to use diffusion in a sentence. A familiar example is the perfume of a flower that quickly permeates the still air of a room.
The mixing of substances due to the motion of their particles. Physics the transmission or reflection of electromagnetic radiation. Diffusion definition is the state of being spread out or transmitted especially by contact. Random movement of molecules across a concentration gradient until the difference in concentration between two regions is near zero a process in which molecules move across a semipermeable membrane until the number of molecules of water is nearly equal on both sides net movement of molecules across a permeable membrane bilayer that requires transport.
Diffusion in british english 1. The dissimilarity in the amounts of solutes particles or molecules between the two regions will cause them to move between the two regions. Diffusion happens in liquids and gases because their particles move randomly from place to place. For diffusion to occur there must be a concentration gradient.
Diffusion is the movement of a substance from an area of high concentration to an area of low concentration. The action of diffusing.